“A pint of sweat will save a gallon of blood.” George S. Patton, Jr.
I intended to begin with “A good plan, well executed, is better than a perfect plan, poorly executed,” but when I checked, that’s not what General Patton said. What he said was “A good plan, violently executed now, is better than a perfect plan next week.”
Somehow, talking about the safety lifecycle in the laboratory as a violently executed plan seemed inappropriate. On the other hand, the effort of sustaining a safe laboratory unquestionably makes a difference and can literally save gallons of blood.
The Safety Lifecycle
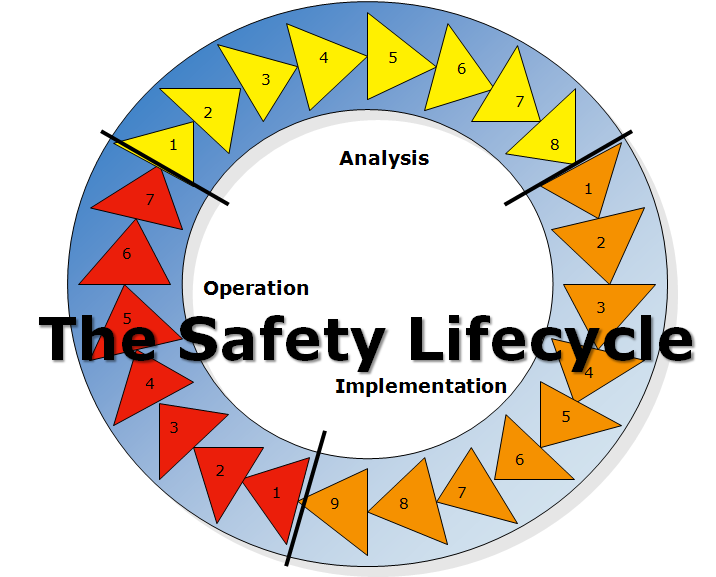
A really useful framework for considering safety is the safety lifecycle, which consists of three phases: analysis, implementation, and operation.
Analysis is the phase of the safety lifecycle where hazards are identified, risk is assessed, and risk reduction measures determined.
Implementation (or “realisation,” for my British colleagues) is the phase of the safety lifecycle where the risk reduction measures are designed, built, installed, and most importantly, where personnel are trained on the operation of those risk reduction measures and on the hazards against which they protect.
Operation, the phase of the safety lifecycle in which the lab spends the vast majority of its time, is where the work of the laboratory is done. The safety tasks during the operation phase of the safety lifecycle include operating safely, testing and inspection, maintenance, continued training, and safe modification and decommissioning.
Most of the literature around the safety lifecycle focuses on automated safety systems for taking a process to a safe state, which are typically known as safety instrumented systems (SIS). An SIS is designed, installed, and cared for by instrument and controls engineers. The safety lifecycle was not the creation of I&C engineers, but it has been championed by them as part of their work with SIS.
For the most part, an SIS is not part of the laboratory environment. Work at the bench scale simply does not rely on automated safety instrumented functions, operated through sophisticated safety logic solvers located in multimillion-dollar control rooms to take a process to a safe state when an unsafe condition is detected. Instead, work at the bench scale relies on safe experimental design and safety-aware laboratory technicians and scientists. Yet, even the laboratory can benefit from applying the principals of the safety lifecycle.
The Analysis Phase
The safety lifecycle graphic shows eight tasks as part of the analysis phase. Adapted to the laboratory, they are
- Experiment design
- Hazard identification
- Risk assessment
- Comparison to risk tolerance criteria
- Risk reduction allocation
- Safety function definition
- Safety function specification
- Reliability verification
A few key ideas should come out of this list: Hazards cannot be identified until there is first a solid idea of what the experiment is supposed to accomplish and how. Risk assessment cannot happen until specific hazards are identified. Risk cannot be zero; there will always be some level of risk and the risk that is “low enough” should be defined in advance. When the risk is not low enough, something must be done about it and since nothing is perfect, it is important to determine how reliable each risk reduction measure can be.
The best means for identifying hazards is a process hazards analysis (PHA). Laboratories are not required to comply with OSHA’s process safety management standard, but it is still a valuable tool and is found in 29 CFR 1910.119. One of the PHA methods listed by OSHA, and the one most likely to be useful in the laboratory, is the checklist. A good resource for laboratory checklists is the 2019 UCLA Chemical Hygiene Plan, published by the University of California-Los Angeles, home of the University of California Center for Laboratory Safety.
The Implementation (Realisation) Phase
The safety lifecycle graphic shows nine tasks in the implementation phase:
- Equipment design
- Software configuration
- Equipment build
- Factory acceptance testing
- Construction/installation
- Site acceptance testing
- Validation
- Training
- Pre-startup safety review
Laboratory equipment needs to be designed, built, installed, and programmed to achieve the safety objectives of the work as well as the experimental objectives. In addition to equipment specific to the experimental program, this includes general equipment such as fume hoods, fire suppression equipment, and safety showers.
Just as importantly, equipment needs to be tested before being put into service. Does each piece of equipment work the way it is supposed to? Do the pieces all work together the way they are supposed to? If there are problems with the design, construction, installation, or programming—and there are always problems—the time to discover them is before hazards are introduced.
The Operation Phase
One of the most insidious problems with safety programs is their misplaced reliance on slogans, like “Safety First.” What does that mean? Get safety out of the way first, so you can get on with the real work? If that’s the case, then the analysis phase of the safety lifecycle should pretty much take care of safety. While most of the literature about the safety lifecycle focuses its attention on analysis, the operation phase is where the safety lifecycle spends the most time.
The safety lifecycle graphic shows seven tasks in the operation phase:
- Operation
- Training
- Proof Testing
- Inspection
- Maintenance
- Management of Change
- Decommissioning
Operating procedures need to address how to do normal tasks safely, and just as importantly, they need to address how to do abnormal tasks safely. Moreover, the best procedures are useless if the personnel using them do not understand them or do not follow them, so training is a continuing requirement.
The two areas of gravest concern in a laboratory, however, are maintenance and management of change.
The UCLA Laboratory Inspection Checklist is an excellent resource for maintaining a laboratory in a safe condition. It is not enough to build a safe experimental program; it has to remain safe. Since everything fails, it important to diligently keep things in good operating condition. The second law of thermodynamics says that broken equipment will not fix itself.
Regarding tests and inspections, it is important to remember that during the time immediately following a proof test is when a piece of safety equipment will work. Either it was working before the test and it will still be working immediately after the test, or it was not working, and IT WAS IMMEDIATELY REPAIRED. Inspection and testing without repair is of no value in keeping a laboratory safe. Failing to keep to the inspection and testing schedule increases the likelihood that equipment will not work as needed.
Management of change is also very important, especially in a laboratory environment. Change is a way of life in the laboratory, and the concerns about managing change do not go away just because it happens all the time. Changes can inadvertently introduce new hazards or inadvertently remove existing safeguards, and so should only be made after considering their impact on safety and health.
Sweat for Blood
The safety lifecycle was not developed for the laboratory, yet it provides a framework that is just as applicable at the gram scale as it is at the railcar scale, even if it differs in the details. Until your laboratory is applying all three phases of the safety lifecycle—analysis, implementation, and operation—to its activities, it is missing out on an easy opportunity to stay safe. Take a look at your safety practices. Have they focused on analysis, while neglecting implementation and operation? Have they concentrated on operation without devoting the time and energy required for good analysis?
If you have not used the safety lifecycle approach before, consider it now. Violent execution is not required. It will take some sweat, but the blood that you save may be your own.
Share Your Experiences
Something that makes safety real for others is shared experiences. You learn from experience, but it does not necessarily have to be your own. In industry, there is a long tradition of exchanging stories about near misses and safety events.
This is not so much the case in the laboratory environment. It is not because near misses and safety events do not occur; we simply do not talk about them. Share your experiences. Comment here if you can. If you are willing, we would be especially interested to hear comments about instances where analysis, implementation, or operational items from the safety lifecycle saved someone in your laboratory from serious harm.
This blog is based on an earlier version, “Three Phases of Safety: The Safety Life Cycle in the Lab”, posted on 26-Jan-2017 by Elsevier in Chemicals & Materials Now!
