“Oxygen is the vital ingredient for the survival of every cell in our bodies. Too little—or too much—can spell disaster.” — Venki Ramakrishnan
A reader recently complained about OSHA’s insistence on defining the safe limits of oxygen in terms of volume percent: 19.5% by volume to 23.5% by volume. “The fact that OSHA does not use partial pressure suggests that their definition is only intended for standard atmospheric conditions.” In regard to the lower safe limit, he noted that “at high altitude, 19.5% oxygen by volume would result in a significantly lower partial pressure of oxygen (PO2) than at sea level, potentially leading to hypoxia.” In regard to the upper safe limit, the reader also noted that “if OSHA intended the 23.5% rule to apply across different pressures, industries would need guidance on how to measure and interpret oxygen enrichment at varying altitudes, which OSHA does not provide.”
Is OSHA oblivious to the difference between volume percent and PO2, or have they simply chosen to assume that all workplaces are essentially at sea level?
Or have they actually addressed extreme conditions that go beyond the limits of 19.5% to 23.5%?
Where Does OSHA Set Oxygen Limits at 19.5% to 23.5%
The limits of a minimum of 19.5% and a maximum of 23.5% are in both OSHA’s Permit Required Confined Spaces Standard in 29 CFR 1910.146(b) and in OSHA’s Respiratory Protection Standard in 29 CFR 1910.134(i)(1)(ii)(A).
The definition of a hazardous atmosphere in the Permit Required Confined Spaces Standard includes “atmospheric oxygen concentrations below 19.5 percent or above 23.5 percent.” To make sure we know they are talking about volume percent, not partial pressure, OSHA goes on to define an oxygen deficient atmosphere as “an atmosphere containing less than 19.5 percent by volume” and an oxygen enriched atmosphere as “an atmosphere containing more than 23.5 percent by volume”.
Interestingly, the definition section of the Respiratory Protection Standard, 29 CFR 1910.134(b) includes a definition for oxygen deficient atmosphere (“an atmosphere with an oxygen content below 19.5% by volume”) but no corresponding definition for oxygen enriched atmosphere. The limits are in the specification for breathing air from atmosphere-supplying respirators, both supplied-air and self-contained breathing apparatus (SCBA): “oxygen content (v/v) of 19.5-23.5%”.
Beyond these two regulations, however, OSHA has more to say about oxygen concentrations in the workplace air we breathe.
At High Altitudes
You don’t have to be a mountain climbing guide to work at high altitudes. Steven Magee, for instance, has written extensively about working at high-altitude astronomical observatories. When the oxygen level is deficient, in other words, less than 19.5% v/v, OSHA requires SCBA or in limited cases, atmosphere-supplying respirators. OSHA links the limits for atmosphere-supplying respirators to altitude. OSHA shows these limits in Table II of the Respiratory Protection Standard, which is also shown below.
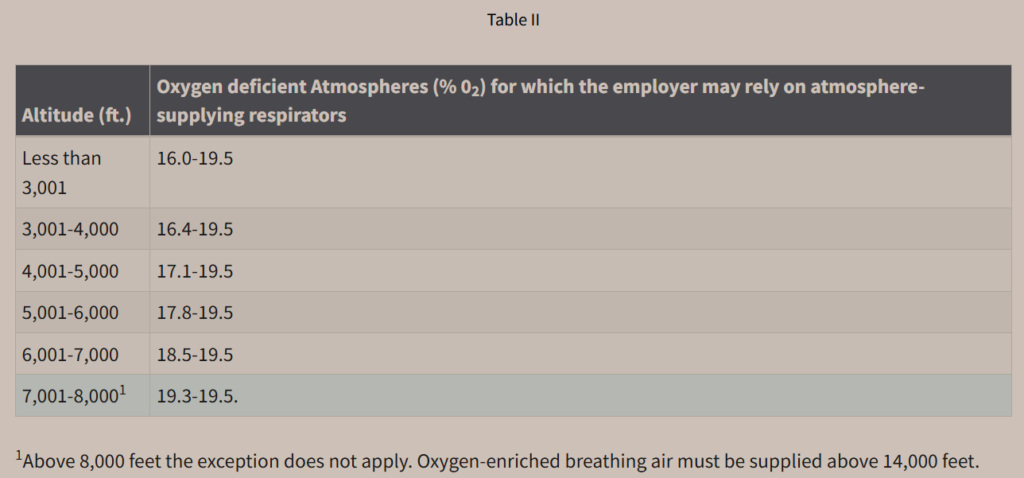
Notice how, as the altitude gets higher, the allowable range of oxygen deficiency narrows, until it reaches 8,000 feet of elevation above sea level. At that point, OSHA no longer permits atmosphere-supplying respirators; instead, OSHA requires SCBA. At an altitude of 14,000 feet or higher, not only does OSHA require SCBA, it requires that the SCBA tanks contain oxygen-enriched breathing air, that is, breathing air with a concentration above 23.5% v/v.
In a 02-Apr-2007 letter of interpretation, OSHA made clear that they considered partial pressure and altitude when they developed the Respiratory Protection Standard:
OSHA recognizes that, at higher altitudes, oxygen in air has a partial pressure that is less than the partial pressure of oxygen in air at sea level; accordingly, the Respiratory Protection Standard makes allowances for employees who work at altitude. OSHA made these allowances based on record evidence showing that such employees usually are acclimated to the reduced oxygen partial pressures and, as a result, will not experience the physiological dysfunction and performance impairments seen in non-acclimated employees. Nevertheless, when the oxygen concentration at altitude becomes oxygen-deficient, paragraph (d)(2)(iii) of the Respiratory Protection Standard requires employers to provide a supplied-air respirator that delivers at least 19.5 percent oxygen to the employee.
Under Water
The other extreme condition that OSHA considers is diving. Subpart T of 29 CFR 1010 is entirely devoted to commercial diving operations. Typically, divers breath compressed air as long as they remain above the decompression limits. Beyond those limits, divers must have access to a decompression chamber.

Photo credit: Volker Lekies on Pixabay, https://pixabay.com/photos/divers-helmet-helmet-diver-205079/
A set of conditions in Appendix C of Subpart T, however, allows for an exception to the decompression rule:
- A nitrogen-oxygen (nitrox) mixture must be used instead of air, and it must contain more than 22% oxygen by volume.
- The nitrox mixture must not exceed 40% oxygen by volume.
- At the pressure being used, the partial pressure of oxygen must not exceed 1.40 atmabs.
(At 40% oxygen by volume, the maximum total pressure is 3.5 atmabs, or 2.5 atmgauge or 36.75 psig. At 22% oxygen by volume, the maximum total pressure is 6.36 atmabs, or 5.36 atmgauge or 78.85 psig.)
The Exceptions Prove The Rules
Has OSHA simply chosen to assume that all workplaces are essentially at sea level?
No. The regulations in the Respiratory Protection Standard, especially as expressed in Table II, make it clear that OSHA doesn’t believe that all workplaces are essentially at sea level and has addressed the extreme conditions of working at high altitudes accordingly.
Is OSHA oblivious to the difference between volume percent and PO2?
No. In the Respiratory Protection Standard’s Table II, OSHA has implicitly addressed partial pressure and in their letter of interpretation about working at high elevations, they explicitly addressed the difference between volume percent and PO2. And in the subpart governing commercial diving, OSHA explicitly uses the partial pressure of oxygen as a basis for their regulations.
Has OSHA addressed extreme conditions that go beyond the limits of 19.5% to 23.5%?
Yes. Under no circumstances is OSHA willing to consider an oxygen concentration less than 19.5% safe. But there are circumstances—working at high altitudes and working under water—where OSHA not only considers but requires oxygen concentrations higher than 23.5%.
Most of us, though, don’t work in those conditions. So, we had better make sure that our breathing air is 19.5% to 23.5% oxygen.
