“In the last two decades of the twentieth century, hundreds of people have died of carbon dioxide asphyxiation near volcanoes in Cameroon and in Indonesia.” — Allison Stark Draper, in Coping With Natural Disasters
Many worry about carbon dioxide in the atmosphere as a greenhouse gas. Carbon dioxide is also a much more urgent hazard. An odorless, colorless gas, it can kill in just a few breaths. Not by global warming, but by asphyxiation.
Tourists staring into an active or dormant volcano’s caldera may see water vapor steaming up out of the crater. But it’s not the water vapor that is deadly, but the gases that they can’t see. This includes carbon monoxide, hydrogen sulfide, sulfur dioxide, and as Allison Draper reports, carbon dioxide.
Carbon dioxide is often described as a simple asphyxiant, like nitrogen or argon, but is it?
Asphyxiants
There are two categories of asphyxiants: simple asphyxiants and chemical asphyxiants (also known as toxic asphyxiants or systemic asphyxiants). Simple asphyxiants are fatal because they are not air and don’t contain oxygen. Chemical asphyxiants, on the other hand, interfere with respiration and the distribution of oxygen to cells. Chemical asphyxiants are fatal even when there is plenty of oxygen available in the air we are breathing.
What Is “Plenty of Oxygen”?
The composition of dry air is
- 78.084% nitrogen
- 20.947% oxygen
- 0.934% argon
- 0.035% carbon dioxide (although this has been increasing)
- Other gases at ppm concentrations or less
OSHA has defined the minimum permissible concentration of oxygen in what we breathe as 19.5%. Below that, we begin to experience negative health effects which get increasingly severe as the concentration of oxygen drops. A level of 6% oxygen in the gas we breathe is fatal, resulting in certain asphyxiation.
A mixture of gases containing 21% oxygen would not lead to simple asphyxiation. An example of “synthetic air” is 80% nitrogen and 20% oxygen. A better approximation to air is a “synthetic air” consisting of 78% nitrogen, 21% oxygen, and 1% argon. Regardless, except for getting the concentration of oxygen at a safe level, the balance of the “synthetic air” could be any mixture at any proportion of simple asphyxiants, e.g., helium, nitrogen, neon, argon, krypton, and xenon.
As long as a gas mixture is at least 19.5% oxygen, it is safe to breath if the balance of the mixture is simple asphyxiants with a total concentration less than 80.5%. If carbon dioxide is a simple asphyxiant, we should be able to breath “synthetic air” composed of 80.5% carbon dioxide and 19.5% oxygen safely.
Chemical Asphyxiants
Chemical asphyxiants have limiting concentrations well below 80.5%. The table shows OSHA’s Permissible Exposure Limit (PEL), the concentration below which it is safe to work, and NIOSH’s Immediately Dangerous to Life and Health (IDLH), the concentration above which it is unsafe to be. It includes recognized chemical asphyxiants, and for comparison, carbon dioxide. It also shows the concentration of oxygen in air that contains the IDLH of the gas.
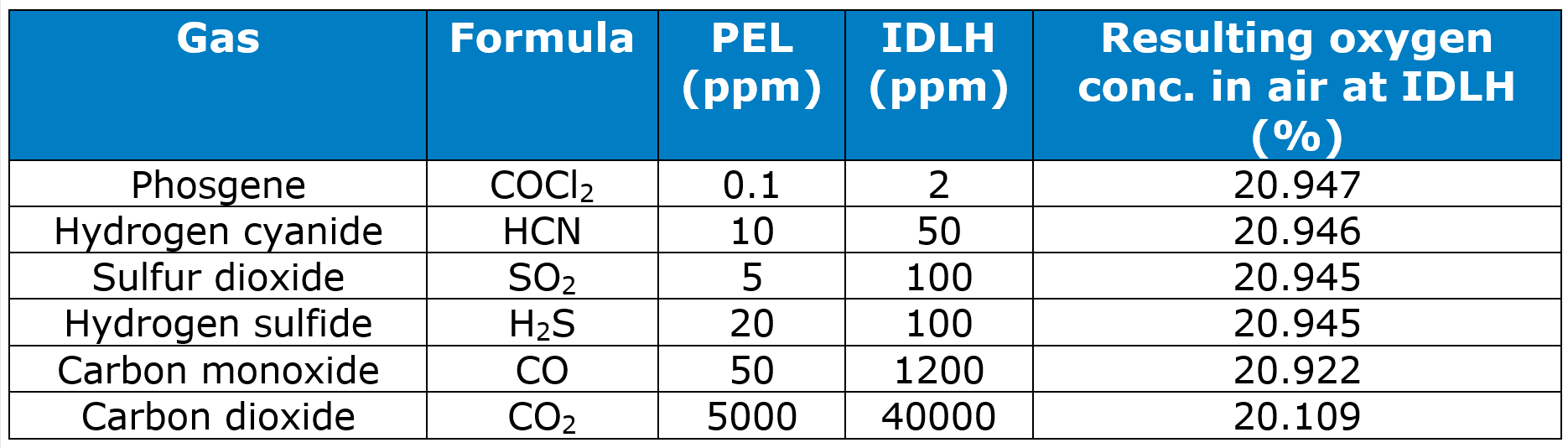
With a PEL that is 100 times higher than the next highest PEL, and an IDLH that is 33 times highest IDLH, it seems that if carbon dioxide is a chemical asphyxiant, it is a mild chemical asphyxiant. It is worth noting, however, that OSHA has not set a PEL and NIOSH has not established an IDLH for helium, nitrogen, neon, argon, krypton, or xenon.
Effects of Carbon Dioxide Poisoning
Many sources describe the symptoms of simple asphyxiation as “rapid breathing, clumsiness, emotional upset, and fatigue. As less oxygen becomes available, nausea and vomiting, collapse, convulsions, coma and death can occur.”
At lower concentrations, carbon dioxide behaves much like simple asphyxiants. A concentration of 0.035% is normally present in the atmosphere, and the concentration in the gas that we exhale is about 4% (40,000 ppm).
At higher concentrations, however, even in pure oxygen, carbon dioxide has different effects. High concentrations cause heart rates, cardiac output, and blood pressure to increase. It also causes hearing and visual hallucinations. Eventually, though, the impact reverses. A 30% CO2 – 70% O2 mixture will cause breathing to stop and convulsions within 1½ minutes.
Using Carbon Dioxide
The most commonly used inerting gas in chemical processes is nitrogen. It is largely unreactive, does not support combustion, and is relatively inexpensive. It’s also a simple asphyxiant. Argon, another simple asphyxiant, is occasionally used as an inerting gas, but it is more expensive than nitrogen. Some facilities use carbon dioxide as an inerting gas. Carbon dioxide, however, is not a simple asphyxiant and its hazards exceed those of simple asphyxiants.
Asphyxiation, whether simple or chemical, results in death. Why does it matter, then, that carbon dioxide is not a simple asphyxiant? The effect of carbon dioxide on our body is out of proportion to the reduction of oxygen levels that would be associated with simple asphyxiants. While not as severe as other chemical asphyxiants, carbon dioxide can surprise us with its toxicity.
