“Hell freezing over? I don’t know. But the devil’s definitely wearing a sweater.” — J.R.Moehringer
Nerd humor requires pointing out that Hell freezing over does NOT equate to sweater weather. Given that Hell is the place where it rains fire and brimstone (sulfur), the temperatures in Hell must be above the freezing point of sulfur. Molten sulfur freezes at 113 C (235°F). So, when it freezes over, it’s colder than normal in Hell, but still not cold enough for the devil to start bundling up.
Many liquids we use here on earth, especially in the process industries, have freezing behaviors that differ from what we typically consider “well-behaved.” Often, they’re either aqueous solutions or mixtures with water. Even water itself isn’t well-behaved. (Ice floats! How weird is that?) Unfortunately, most of our experience with freezing liquids is with ice, so most of us unconsciously use water as our point of comparison.
The safety of process facilities often depends on knowing the freezing behavior of the liquids they are using. An inadequate understanding can lead to inadequate freeze protection, and an unexpected loss of flow can lead to catastrophic failures, both operationally and in terms of safety.
Freezing Point Depression
A commonly observed phenomenon in aqueous solutions and mixtures with water is freezing point depression. As the concentration of the lesser component increases, the freezing point of the solution or mixture drops. Here are some examples:
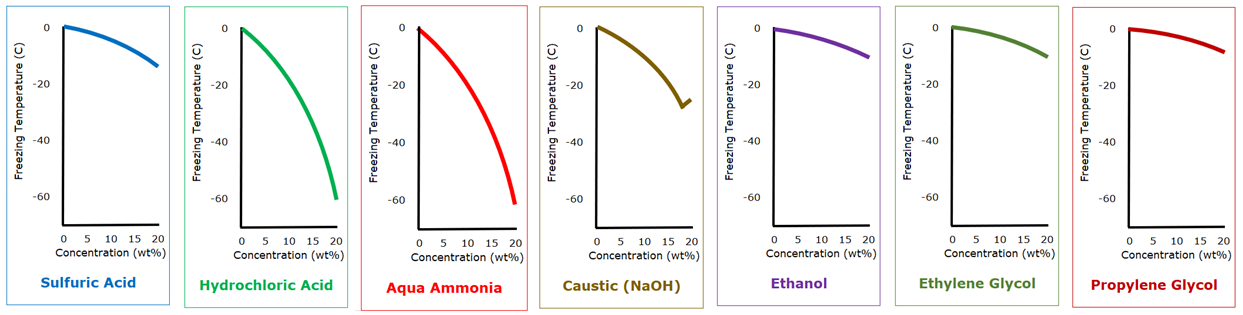
With relatively dilute solutions, about anything can be used as antifreeze for water. The advantages of the glycols is not their superior freezing point depression as dilute solutions, but their relatively high flash point (which happen to above 100 C, 212°F) and their relative non-corrosivity. Except that they grab you by the throat and don’t let go, it appears that aqua ammonia or hydrochloric acid would be better choices for antifreeze.
Antifreeze
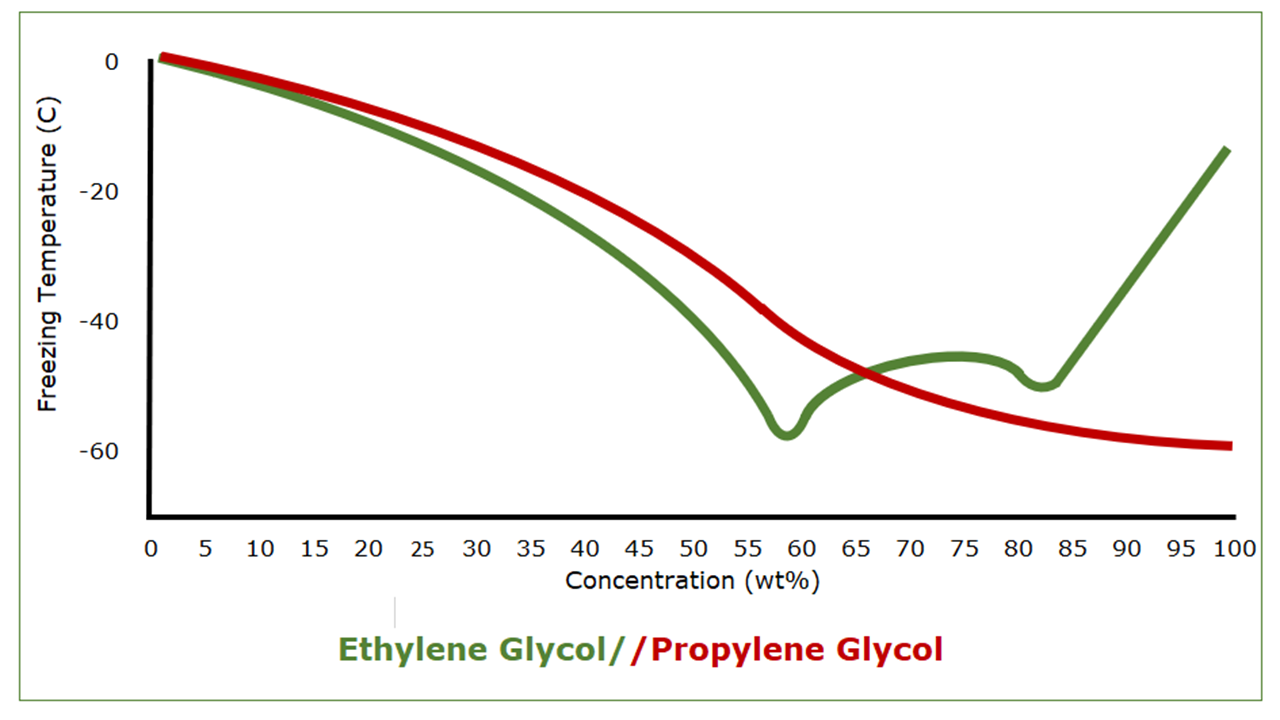
An interesting thing about ethylene glycol and propylene glycol, though, is what happens as their mixtures with water get more concentrated.
Pure propylene glycol has a freezing point of -59 C (-74°F). As mixtures of water and propylene glycol get stronger, the freezing point drops, albeit not steadily, from that of water to that of propylene glycol. Pure ethylene glycol, on the other hand, has a freezing point of -13 C (9°F). Ethylene glycol/water mixtures provide better freeze protection than predicted by their freezing points, however, because of a eutectic freezing point of -49 C (‑57°F) at 59 wt%. This is why auto manufacturers recommend a 50/50 mixture. It’s not to save on ethylene glycol.
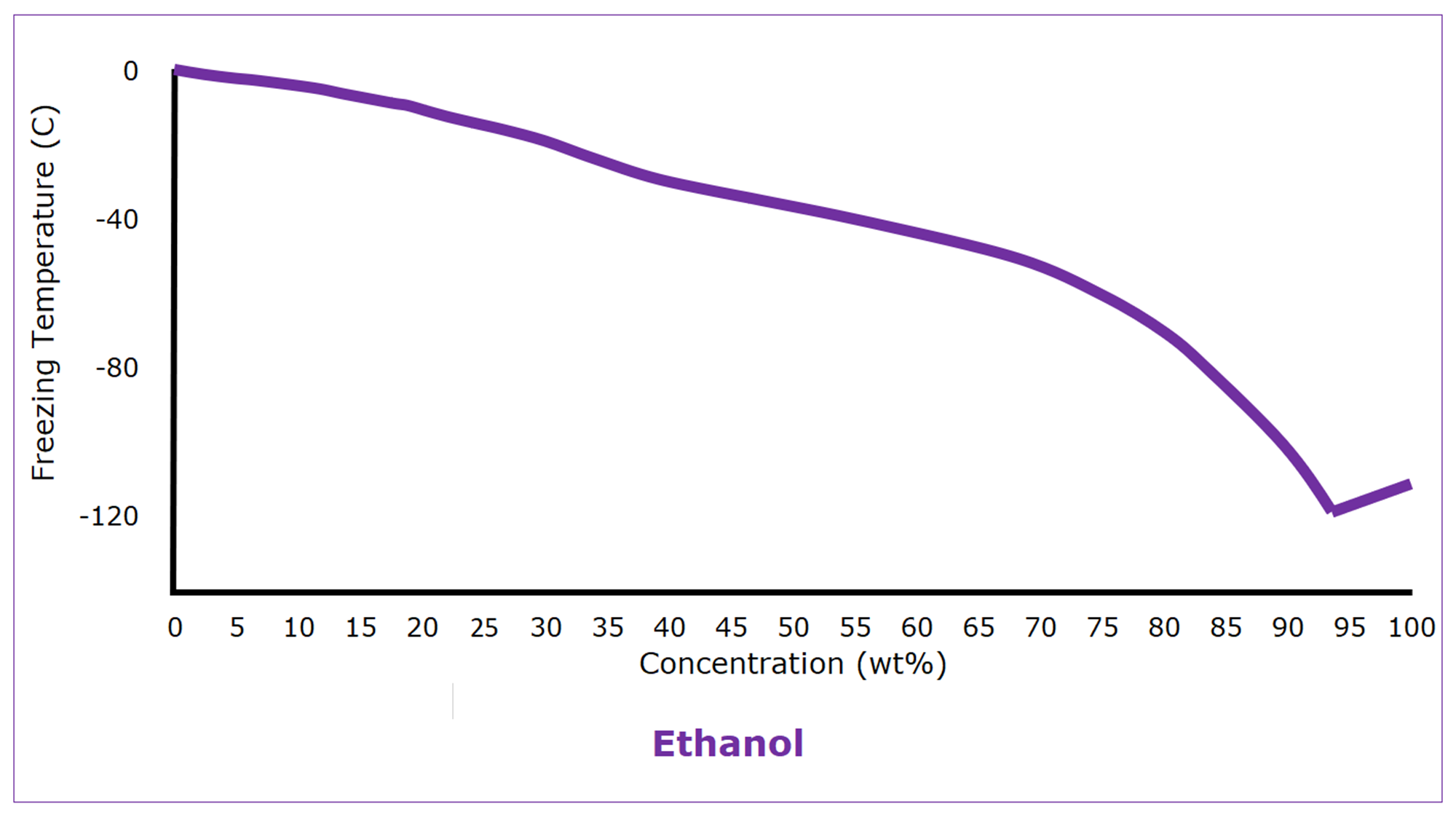
Alcohols are used as antifreeze as well. (Windshield wiper fluid is 30% methanol in water.) Ethanol, for instance, is completely miscible with water and has a freezing point of -114 C (‑173.5°F). If it weren’t for its easy flammability, we would use ethanol as antifreeze more often. Ethanol-water mixtures have reasonably well-behaved freezing properties, but even ethanol-water mixtures have a modest eutectic freezing point of -119 C (‑182°F) at 93 wt%.
This discussion of the behavior of water-miscible organic liquids does little to prepare us to anticipate the behavior of inorganics solutions in water. Consider two commonly used acids, hydrochloric acid and sulfuric acid, and two commonly used bases, ammonium hydroxide and sodium hydroxide.
Hydrochloric Acid
Hydrogen chloride is a gas at standard conditions. It freezes at -114 C (‑173.5°F), coincidentally the same as ethanol. Its freezing is nothing like that of ethanol, however. Hydrogen chloride doesn’t become hydrochloric acid until it dissolves in water, where it completely dissociates. As an aqueous solution, the behavior of hydrochloric acid isn’t unexpected: increased concentration depresses the freezing point. But then there is a eutectic freezing point of -75 C (‑103°F) at 23 wt%. At higher concentrations, the freezing point is higher, although hydrochloric acid goes through three more eutectic freezing points before it gets so concentrated (62 wt%) that the solution can dissolve no more hydrogen chloride gas.
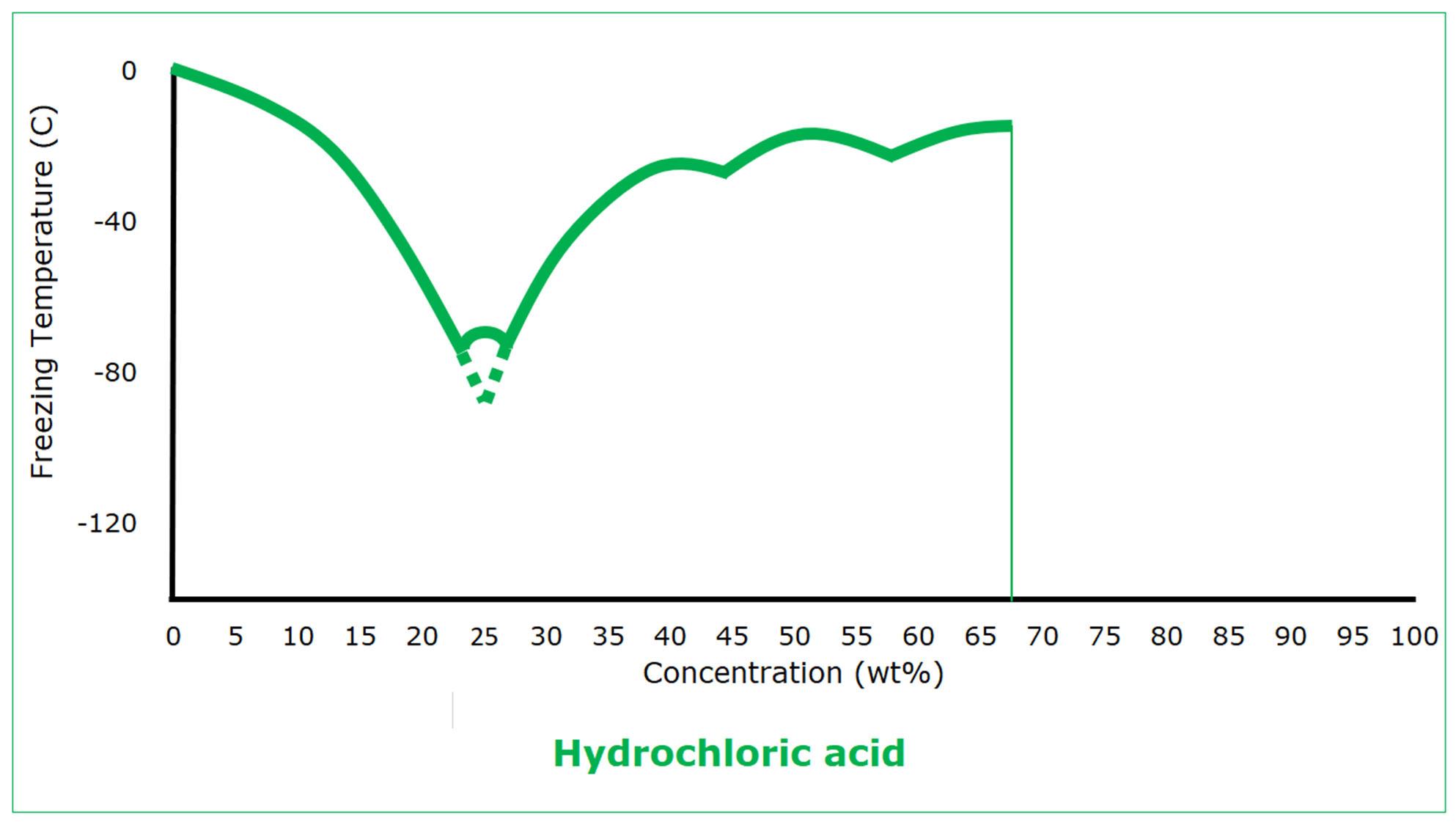
Once the concentration of hydrochloric acid is higher than that of the first eutectic, the freezing point behavior as a function of concentration is not simple, but it never exceeds the freezing point of water. The protection that can be used to prevent water from freezing will also prevent hydrochloric acid from freezing.
Sodium Hydroxide
We typically refer to aqueous solutions of sodium hydroxide, or lye, as “caustic.” Unlike hydrochloric acid, which is a gas dissolved in water, caustic is a solid dissolved in water. Pure lye has a melting point of 318 C (604°F). Caustic solutions follow the expected behavior of freezing point depression at low concentrations. At a concentration of 19 wt%, however, the freezing point starts climbing, although not in a way that a general understanding of freezing point behavior would predict.
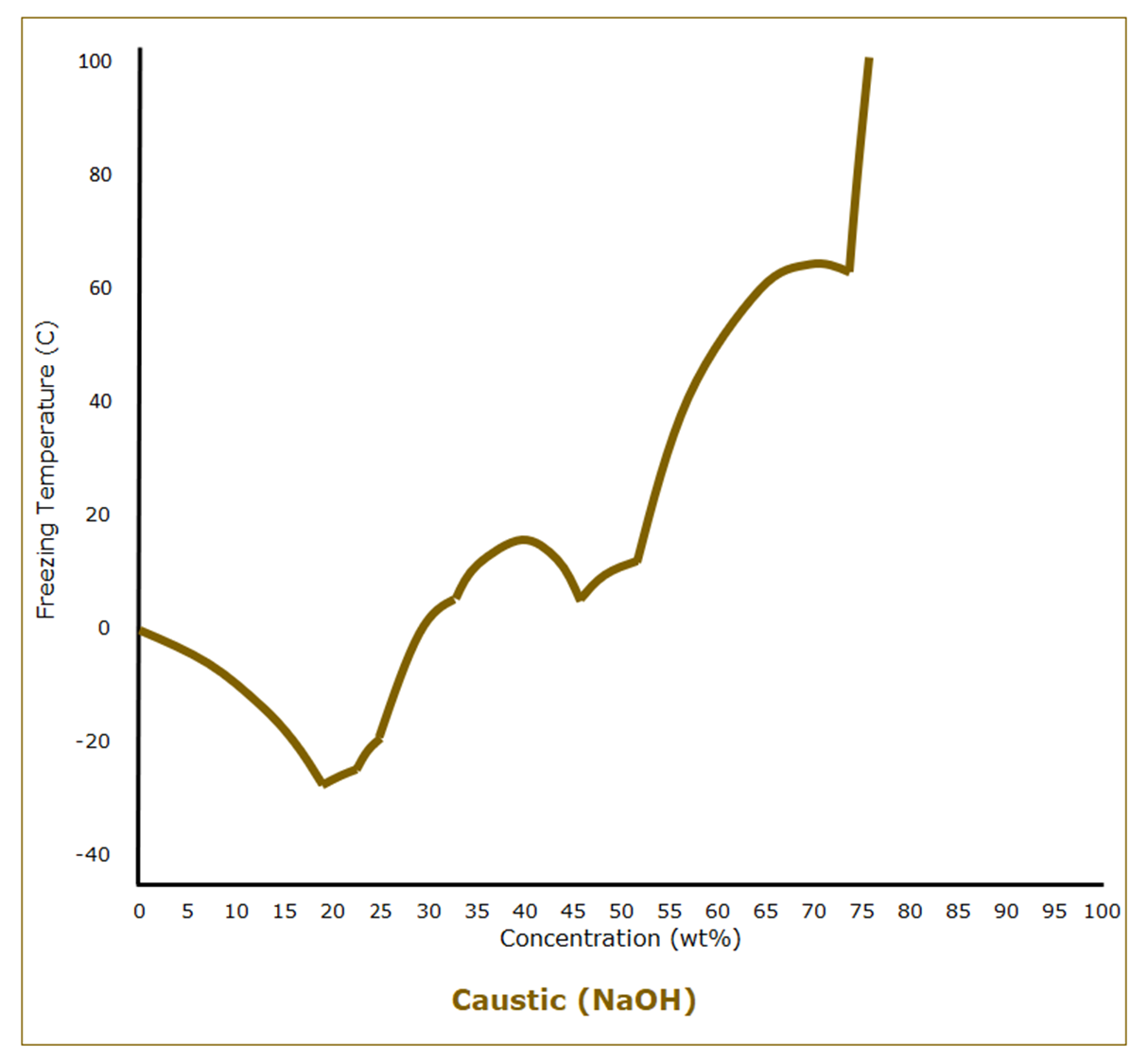
Even knowing that the freezing point of 50 wt% caustic, a commonly used grade, is 14 C (58°F) isn’t much help in understanding the entire freezing point curve of caustic and the potential complexities of freeze protection.
Ammonium Hydroxide
Ammonium hydroxide, or aqua ammonia, results from the reaction of ammonia, a gas at standard conditions, with water. In the absence of water, ammonium hydroxide cannot exist. Dilute aqua ammonia solutions behave the way we expect, depressing the freezing point as the concentration increases. That is, until the concentration reaches 33.4 wt% as ammonia (68.7 wt% as ammonium hydroxide) which has a eutectic freezing point of -100 C (‑148.5°F). At even higher concentrations, aqua ammonium goes through two more eutectic freezing points, but generally, the freezing point stays in the range of about -100 C to -80 C (about -150°F to ‑110°F). Then, when the ammonia concentration is 100%, the freezing point is that of anhydrous ammonia, -77.6 C (‑107.7°F).
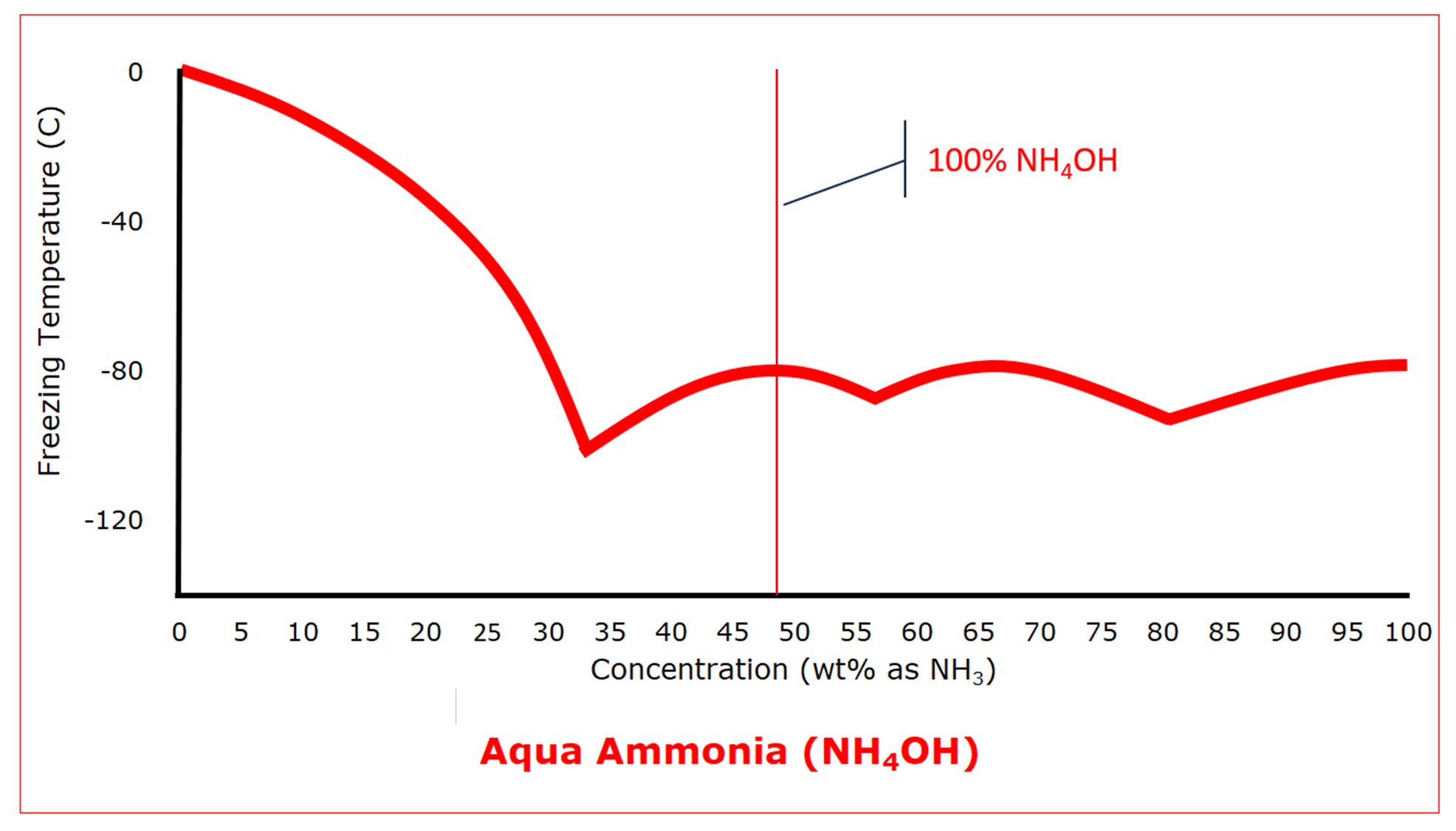
As was the case with hydrochloric acid, the protection that can be used to prevent water from freezing will also prevent aqua ammonia from freezing.
Sulfuric Acid and Oleum
Like ammonium hydroxide, which is the reaction product of ammonia and water, sulfuric acid is the reaction product of sulfur trioxide and water. It does not exist in the absence of water. And like ammonium hydroxide, where there can be solutions with an excess of ammonia, sulfuric acid solutions can exist where there is an excess of sulfur trioxide. We call these “oleum.”
Most of us think of sulfur trioxide as a gas because of the high temperatures at which it is produced, but it condenses to a liquid at 45 C (113°F). It exists as a solid in three forms; the most relevant are the beta form, with a freezing point of 32.5 C (90.5°F), and the gamma form, with a freezing point of 16.8 C (62.2°F).
Sulfuric acid, like everything else this blog considers, starts off depressing the freezing point as the concentration increases, until it reaches its first eutectic freezing point of -61 C (‑78°F) at 29.4 wt% SO3 (36% sulfuric acid). From there, it is a roller-coaster ride as it climbs to the freezing point of the beta form of SO3.
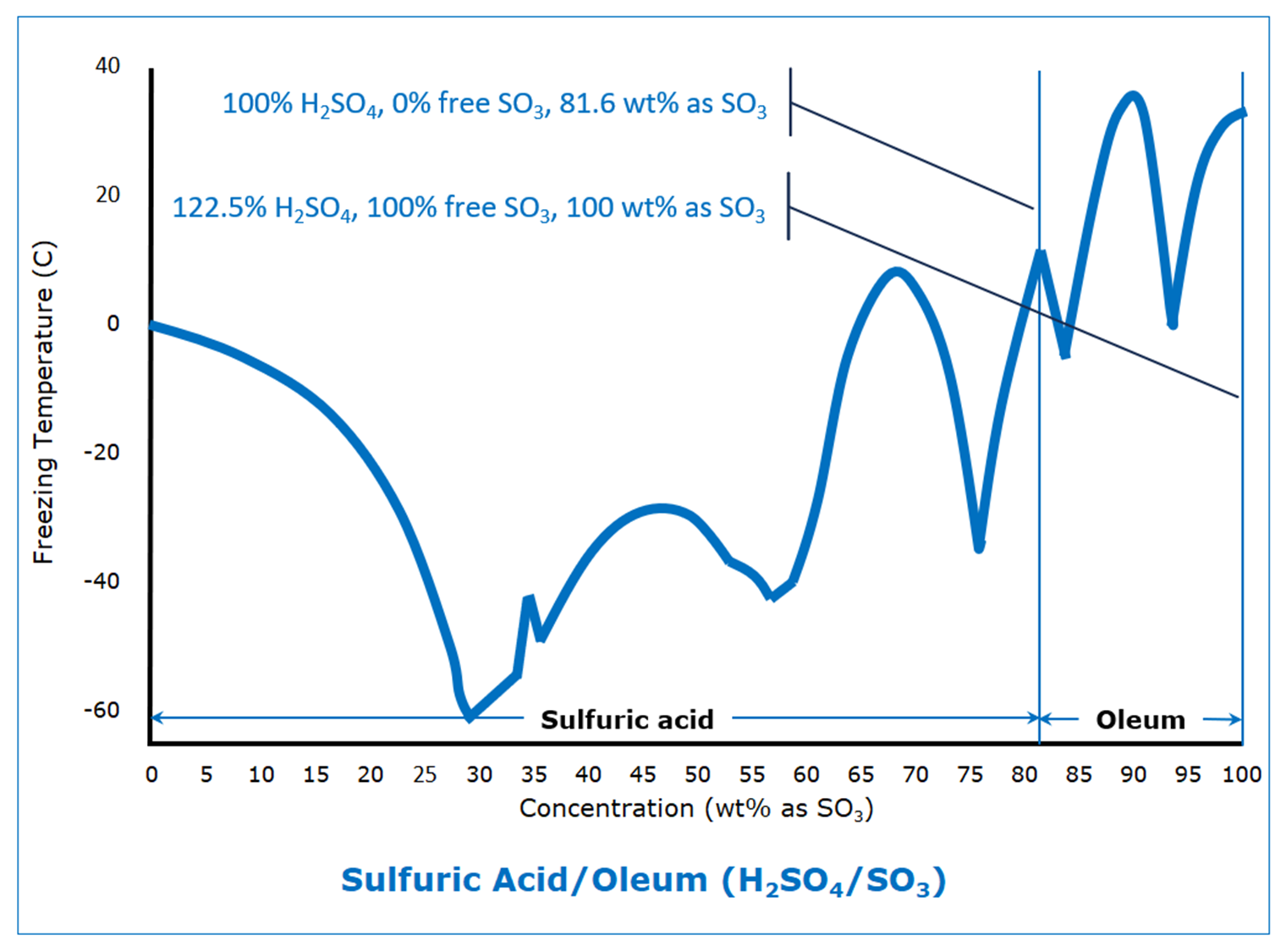
It is that roller coaster ride that can cause freezing problems for the unsuspecting. A slight shift in concentration can result in huge shifts in freezing points, up or down, which can lead to unanticipated hazards.
Ain’t Misbehavin’
No one should expect that the freezing point of aqueous mixtures and solutions should be a matter of connecting the dots. You can’t simply plot the freezing point of water on the left side of the graph and plot freezing the point of the other component on the right side of the graph, then draw a line between the two points. At the very least, there will be freezing point depression. At some point, the freezing behavior departs from any simple understanding of freezing behavior. It’s not misbehavior, it’s just what it is.
This means that for processes involving aqueous mixtures and solutions, a hazard evaluation must include process safety information on the freezing behavior of those solutions, and the impact of concentration on that behavior.