“Emotions can certainly be misleading: they can fool you into believing stuff that is definitely, demonstrably untrue.” — Francis Spufford
Places that rarely experience snow and are ill-prepared to deal with it have seen debilitating snow storms this year. In response, broadcast meteorologists and local news anchors offered advice on choosing ice melt that was safe.
Safe for what? Among the list of concerns were pets, the environment, and concrete driveways. At the top of the list of “safe” ice-melts, at least according to the broadcasters I watched, was “magnesium”. Magnesium wasn’t a salt, they said, so it was safe.
I assumed that they meant magnesium chloride, which most assuredly is a salt. In fact, most ice melts are compounded with salts. But their advice does beg the question: is there a safe ice-melt?
Common Ice Melt Formulas
Several chemicals are offered as ice-melt compounds. The most familiar and least expensive, of course, is rock salt—sodium chloride (NaCl). The most common ingredients in ice-melt formulations are
- Sodium chloride (rock salt) – NaCl
- Potassium chloride – KCl
- Calcium chloride – CaCl22 H2O (In the solid form, calcium chloride is typically a dihydrate.)
- Magnesium chloride – MgCl2n H2O (Hydrated magnesium chloride can vary from n = 1 to n = 12, although n = 6 is the most common)
- Potassium acetate – KOOCCH3 (the potassium salt of acetic acid)
- Calcium magnesium acetate (CMA) – CamMgn(H3CCOO)2m+2n (CMA can consist of any ratio of calcium to magnesium, but the Federal Highway Administration’s 1986 investigation determined that the ideal Ca:Mg mole ratio was 3:7.)
- Urea (carbamide) – CO(NH2)2 (Typically produced by the reaction of ammonia with carbon dioxide, urea is the only ice-melt of these seven that is not a salt.)
Safe For Pets?
PetMD identifies three types of health problems for pets when exposed to ice melts: topical, gastrointestinal, and neurologic. Humans can also suffer from these problems; we’re just less likely to groom ourselves by licking the ice melt off our skin.
Topical hazards – Exposure of eyes, nose, mouth, skin, and paw pads can result in irritation. The chloride salts are more severe than the acetates. Urea is the most benign, but it is not harmless.
In addition to irritation, there is also a concern with the heats of solution. When a solid dissolves in water, such as the sweat on your hand or the tears in your eyes, it will either release energy (exothermic) or absorb energy (endothermic). In the case of calcium chloride, the amount of energy released can be enough to cause burns.
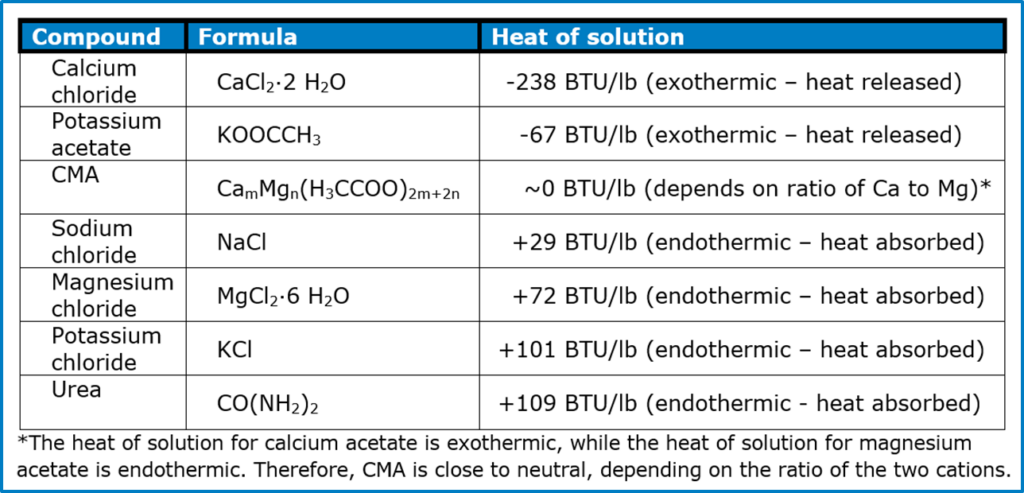
It should come as no surprise that many chemical hot packs use calcium chloride. The amount of heat release is even more if the salt is anhydrous. Likewise, urea is occasionally used in some chemical cold packs.
Gastrointestinal hazards – Excess amounts of any salt can throw electrolytes out of balance. On a per-pound basis, chlorides are worse than acetates, and sodium is worse than potassium, calcium, and magnesium. There is also the well-known link between sodium and hypertension (high blood pressure). None of the other cations have this effect.
The folks at Small Door Veterinary report ice melts can cause these symptoms when consumed:
- Diarrhea
- Vomiting
- Tremors
- Seizures
- Dehydration
Ice melt can be fatal when consumed in large enough quantities. The oral toxicity of ice melts is low for all seven, in the range of 2 to 3 grams per kilogram. Two ice melts are even less toxic: magnesium chloride and urea, each with an oral toxicity of around 8 grams per kilogram. Compare these to the LD50 of antifreeze, ethylene glycol, which is 1.3 grams per kilogram.
Neurologic hazards – These are typically the least of the concerns with ice melts. Excess amounts of sodium salts can lead to hypernatremia which has neurologic symptoms: confusion, lethargy, seizures, and unconsciousness, among others.
Safe for the Environment?
All salt-melting compounds have an impact on the environment, usually not positive. Chlorides impact vegetation much like drought, causing leaves to brown and drop off, stunted growth, and dying limbs. Chlorine is not biodegradable and is very soluble, meaning that it lingers in the environment for a long time, continuing to do harm.
The cations—sodium, potassium, magnesium, and calcium—are also not biodegradable. Potassium, magnesium, and calcium are vital plant nutrients, but in excess, will release heavy metals that would normally stay bound to soil particles. Sodium and potassium increase the salinity of soil and decrease soil’s permeability.
Acetate is biodegradable and improves the growth of roadside plants. When it washes into surface waters, however, it reduces the dissolved oxygen in surface water as it decomposes, which has a mild negative impact on aquatic animal life.
Urea’s principal use is as a fertilizer. As such, it can have a generally positive impact on the environment in small amounts. In large amounts, however, it promotes algae growth and all the problems associated with that.
Safe for Concrete and Equipment?
In addition to the harm that ice melts can do to our pets and our environment, there is also a very real concern about the harm they do to the concrete surfaces they are deicing and to the vehicles operating on those surfaces.
Chlorine leads to chloride stress corrosion cracking in metals. Any chloride salt will accelerate corrosion in most commonly used metals, including stainless steel. Neither acetates nor urea are free from corrosion hazards, but they are minimal, especially when compared with chlorides. As for the cations, they have little influence on metal corrosion at all.
Concrete damage also happens. Typically, it is the freeze/thaw cycle that does this, not the ice melt compound in the resulting brine. If the damage to the concrete goes down to the reinforcing steel, then chlorides will accelerate the corrosion of the steel, accelerating spalling.
The Effectiveness of Common Ice Melt Compounds
There are many ice melts on the market, many of which claim to be “pet-friendly” or “eco-friendly”. Before considering the safety of a compound as an ice melt, it must be effective at melting ice. Dirt may be safe on all counts, but it doesn’t melt ice.
Ice melts work by lowering the freezing/melting temperature of the ice/water/brine. A little bit of salt lowers the melting temperature a little. More compound lowers the melting temperature more, until the resulting brine reaches a concentration that gives the lowest possible melting temperature. This is the ice melt’s eutectic temperature.
The eutectic temperature for NaCl is -6°F, at a concentration of 23.3 wt%. Sodium chloride is only considered effective down to 15°F, however. To get all the way down to the eutectic temperature of -6°F, 23.3 pounds of salt can only melt 76.7 pounds of ice. In other words, one pound of NaCl will melt only 3.3 pounds of ice. That is simply not economical. Below ‑6°F? NaCl simply will not melt any ice.
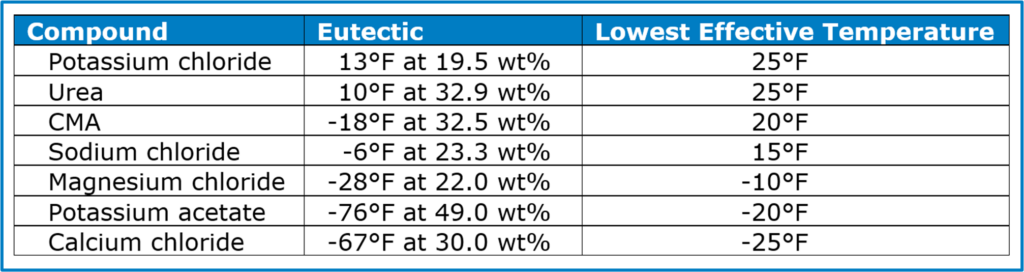
Neither potassium chloride nor urea are much more effective than dirt. The package of pet-safe urea ice melt that I have is going on my garden, not my driveway. The other chlorides are effective, as is potassium acetate. CMA, the new darling of the ice-melt world, is moderately effective.
Avoid Rock Salt and the Other Chlorides
If all you care about is melting ice, then nothing beats rock salt, which is the cheapest, most readily available of all the ice melt compounds. But it is the worst in terms of pet safety, environmental safety, and corrosion. On the other hand, potassium acetate causes the least harm in terms of pet safety, environmental safety, and corrosion, and it is very effective. You will have to go out of your way to find it, though.
The wisest course of action is to avoid rock salt and the other chlorides and vary the compounds that you use throughout the winter season, so no one compound dominates. And with all ice melts, use them sparingly. That will be safer and save money. Then, after the pavement is dry, sweep up any ice-melt that remains so it is not there to harm your pets, the environment, or your pavement. Save it for the next snow.
