“They swore by concrete. They built for eternity.” — Günter Grass
A tank truck hauling gasoline caught fire under an I-95 overpass outside of Philadelphia on Sunday, June 11, 2023. Not long after, the overpass collapsed. Concrete is not combustible, and most of us don’t think of structural steel as combustible, either. So, how could this happen? How could a burning truckload of gasoline cause a concrete structure to fail?
“Not Combustible” Doesn’t Mean “Fireproof”
Decades ago, I was on a tour of a three-story factory that was the property of the city because of tax foreclosure. The city was trying to interest anyone in redeveloping the property into something that would contribute to the city’s tax base. It was a brick building internally supported with massive wood timbers. The fire chief was part of the tour, and I commented that despite the brick construction, the internal wood structure must really give him pause.
His answer surprised me. “No, I would rather fight a fire in this building than in one built with steel or concrete. All three of them will fail in a fire, but steel and concrete both collapse catastrophically and with no warning. The char that forms on these huge timbers will actually slow down the rate they burn through, which gives us time to put the fire out.”
Until then, I had always thought of steel and concrete as “fireproof”. They’re not.
Steel Isn’t Fireproof
Concrete structures, like dams, high-rises, and highways, use a lot of steel. That includes the rebar that lends tensile strength to the concrete, as well as the steel girders that are sometimes used to support the concrete deck of a bridge. Steel oxidizes. When this happens slowly, we just call it “rusting”. As temperatures get higher, the oxidation gets faster, until the steel is burning. It doesn’t burn with flames, but in a shower of incandescent sparks. If you’ve ever seen a cutting torch at work, that’s what’s happening: the jet of extra oxygen turns a heating torch with a slightly reducing atmosphere to a cutting torch with a strongly oxidizing atmosphere.
“Fireproofing” structural steel means insulating it with something that can withstand the temperatures of a fire. Not so much to protect the steel from oxidation, but to slow down the heat transfer. Because as steel gets hotter, it loses its strength.
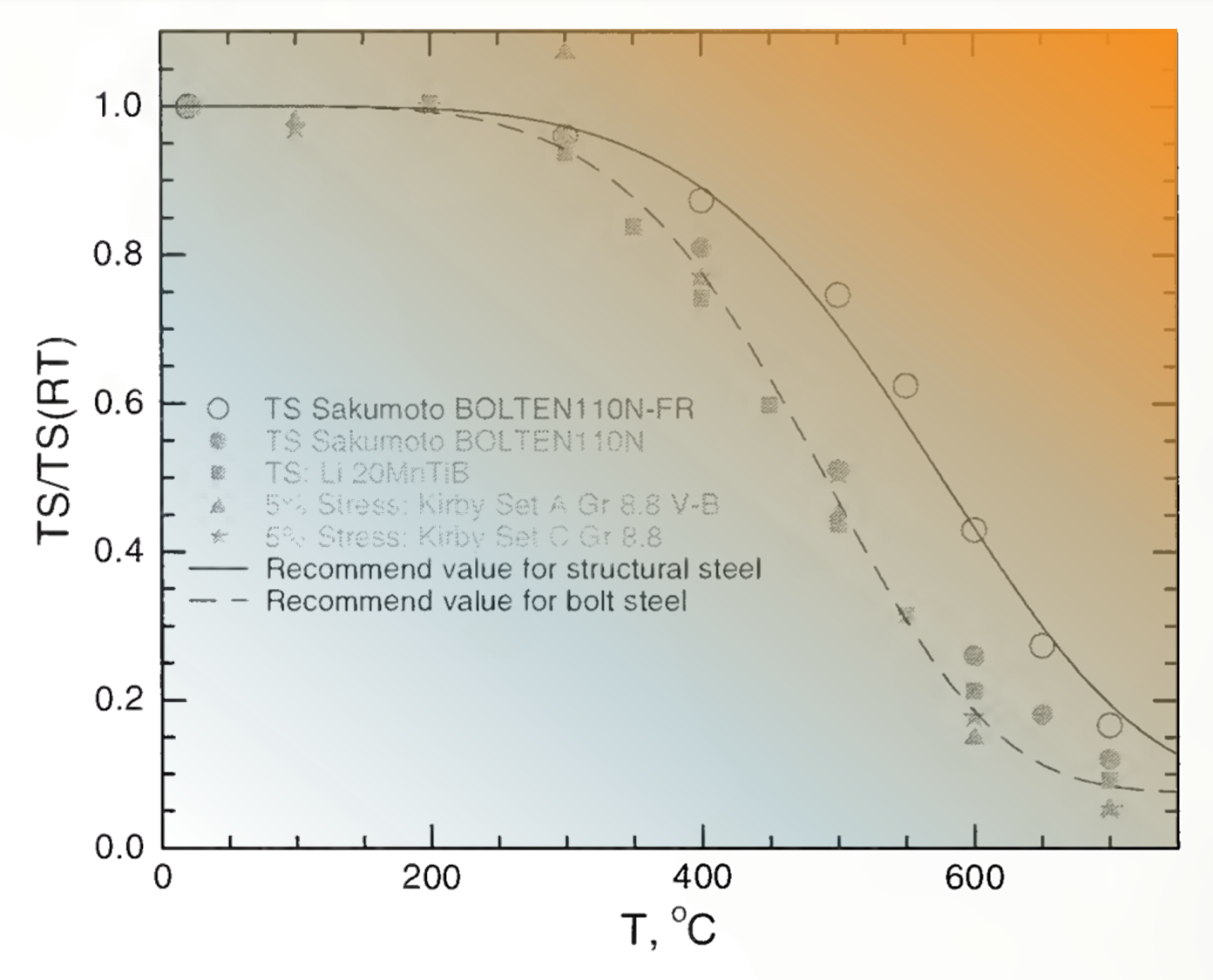
Figure 1. The relative weakening of tensile strength (TS) of structural steel as it heats.
Following the September 11 attack on the World Trade Center in New York, the National Institute of Standards and Technology published a report, Mechanical Properties of Structural Steel, that included the graph shown in Figure 1. The strength of structural steel remain largely unchanged until it reaches about 300 C (about 570°F). Then it begins to drop, slowly at first but losing most of its strength by about 700 C (about 1,300°F), at which point it glows a bright red-orange. This is long before steel reaches its melting range of 1,370 to 1,540 C (2,500 to 2,800°F). This weakening occurs because of the temperature, not because of exposure to air. For a time, embedded rebar is protected because concrete acts as insulation against heat transfer.
Concrete Isn’t Fireproof, Either
Unlike steel, which is a homogeneous solid solution of iron, carbon, and other metals, concrete is a heterogeneous mixture of a filler (aggregate) and binder (cement). When concrete fails, it’s because the binder failed.
Modern concretes use Portland cement as their binder. Portland cement is mixture of about 50% tricalcium silicate ((CaO)3·SiO2) and 25% dicalcium silicate ((CaO)2·SiO2), with lesser concentrations of tricalcium aluminate, tetracalcium aluminoferrite, and gypsum (CaSO4·2H2O) making up the balance. It’s not until the dry mixture reacts with water, however, that Portland cement can bind aggregate into concrete. At first, the mixture of Portland cement and water forms a paste, but with time, the water reacts with the components of the cement.
This reaction with water — hydration — is a set of reversible reactions, the most important being those of tricalcium silicate and dicalcium silicate:
2 (CaO)3·SiO2 + 7 H2O ßà (CaO)3·(SiO2)2·(H2O)4 + 3 Ca(OH)2 + 173.6 kJ
2 (CaO)2·SiO2 + 5 H2O ßà (CaO)3·(SiO2)2·(H2O)4 + Ca(OH)2 + 58.6 kJ
The tricalcium silicate hydrate forms a gel within the paste that binds with aggregate, while the calcium hydroxide crystalizes in the voids between aggregate, binding it. A lot of heat is generated in the process. When the concrete cures, the water is reacting, not evaporating, which is why it is important to keep concrete from drying out as it cures.
In a fire, the reactions reverse, as the heat drives off the water in a dehydration reaction. The kinetics are complex, but work by using the energy of the fire to force the endothermic reaction. Qi Zhang and Guang Ye studied the dehydration of cured Portland cement past and developed thermogravimetric curves as a function of temperature:
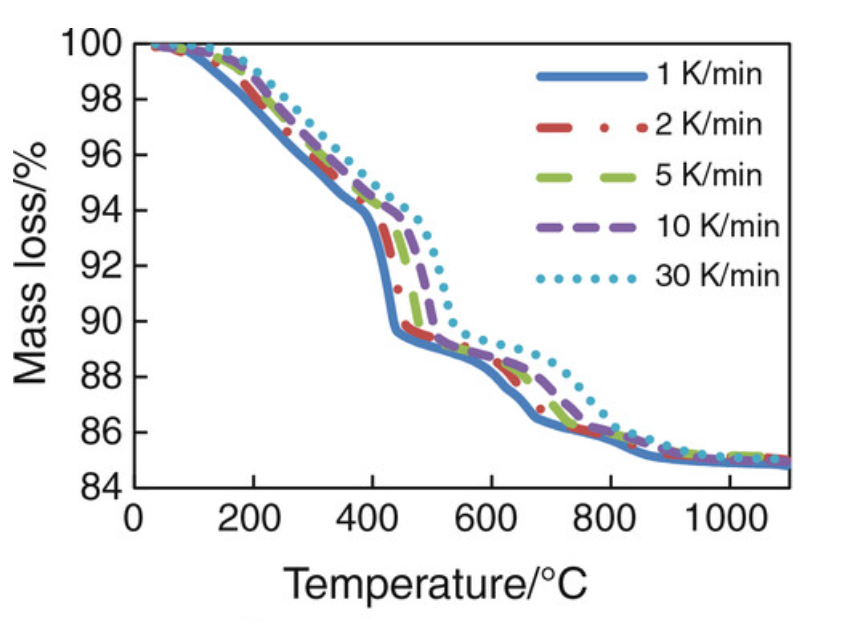
Figure 2. Dehydration kinetics of Portland cement paste at high temperature.
The lost mass is water. The cement loses mass throughout a ramped heat-up until about 900 C (1,650°F), when the water of hydration is all gone. Importantly, there is a sudden loss in mass as dehydration gets fully underway in the range of 400 to 500 C (750 to 930°F), when the cement fails. The slower the paste is heated, the lower the temperature that the dehydration occurs. More interesting is that the temperature at which the cement fails is very similar to the temperature at which structural steel fails.
How Hot Does Gasoline Burn?
The adiabatic flame temperature of a stoichiometric mixture of gasoline and air is about 2,140 C (3,880°F). This is under ideal conditions, where the gasoline vapors and air are well mixed, and the flame is clear blue and not smoky. Actual conditions for burning gasoline, especially if the gasoline is spilling from an overturned tank truck, are less than ideal for maximizing flame temperature.
Under actual conditions, the flame temperature of burning gasoline is less, typically around 1,030 C (1,880°F). Flame temperatures for other burning materials differ. Charcoal flames, for instance, are in the range of 750 to 1,200 C (1,400 to 2,200°F), while animal fat flames are in the range of 800 to 900 C (1,450 to 1,650°F) and uncontrolled natural gas flames are in the range of 900 to 1,500 C (1,650 to 2,750°F).
So, actual gasoline flame temperatures are much cooler than the theoretically possible temperature. The burning gasoline flames under the I-95 overpass were considerably cooler than well-controlled adiabatic flames. The heavy deposit of soot not only shows that the flame was smoky, but that the fuel-air mixture was fuel rich – not stoichiometric. Nonetheless, actual fires are still hot enough to weaken structural steel and to dehydrate the cement binding the concrete. When the concrete and the structural steel both fail, there is nothing to hold a structure together.
Sustained Fires Will Also Bring Down Process Structures
It’s not just transportation infrastructure that depends on steel and concrete. The structure that supports process equipment is also built from steel and concrete. Our experience tells us that both will deteriorate with time, but many of us struggle with the misconception that they are fireproof, that any failure will come with warning. However, a sustained fire over 600 C (1,100°F) will bring those structures down, even though they aren’t burning. And flames at over 600 C (1,100°F) can be achieved in actual conditions by just about anything that can burn.
Concrete and structural steel maintain their strength if they can be kept cool, typically at less than around 400 C (750°F). “Fireproofing” insulation will slow the transfer of heat into the structural supports but will not stop it. Monitors and other cooling sprays will absorb the heat before it can flow into the structures.
Be Wary of Structures that Have Experienced a Fire
Concrete and, to a certain degree, steel are non-combustible, and so, fire-resistant. They are not, however, fireproof. Consider which structures in your facility are vulnerable to a sustained fire and determine if appropriate fireproofing measures have been applied.
If there is a fire, be wary of the structure after the flames have been extinguished. A sustained fire can weaken the structure even if it doesn’t bring it down. Be sure to have the structure evaluated before attempting to put it back into service.
